Hard Water
Hard Water
If, on its way to your tap, your water passes through rock with a high calcium or magnesium content such as limestone, coral stone or chalk (and incidentally cement/concrete) it will pick up calcium or magnesium which dissolves in the water. The water becomes “hard”. So what is hard water? How do we know if our water is hard and what can we do to make it soft? Here we look at the symptoms and problems that hard water causes, particularly the deposit of scale, and we look at how we can use a water softener to remove the dissolved calcium to make the water soft.
See also:
- Water causes stomach problems
- Water contamination and how it may be caused
- Water laboratory testing and how to read the results
- How to filter your water and contamination that can be removed
What Is Hard Water?
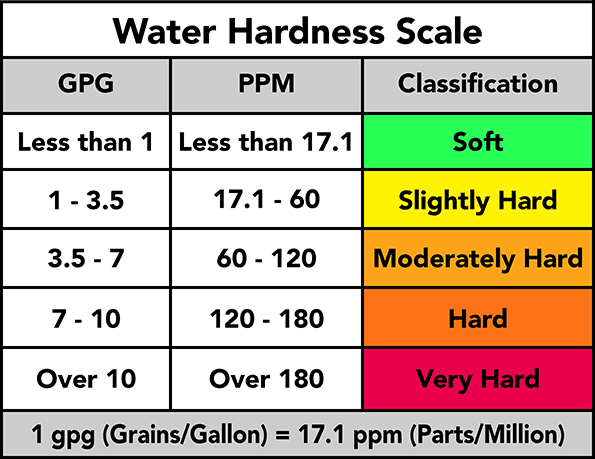
If you are unfortunate enough to live in an area where water is drawn from lime rock you will probably find your water is hard. The hardnes is caused by calcium or magnesium dissolved in the water. As water perculates through limestone (or chalk) traces of the rock are dissolved by the water which leaves the water feeling hard.
How do I know if my water is hard?
Symptoms of hard water are as follows:
- You cannot get soap to lather
- The water feels "hard" when you wash in it.
- Hard water will leave white deposits anywhere that have become wet and the water has dried off such as glass shower partitions, glasses, crockery and cutlery, wall and floor tiles in bathrooms, in toilet bowls, around taps, and in kettles and water heaters.
- Hard water can also leave our clothes stiff, the stiffness will increase over time as more calcium is left in the fabric.
You can get your water tested to find out if your water is hard and how serious is the problem.
Hard Water Causes Scale
When hard water gets some rough treatment such as being heated or running through something or dripping onto something it can deposit the calcium/magnesium. Deposits can build up quite quickly, visit a show cave and you will soon see what deposited calcium can do.
Around your house calcium or magnesium can get deposited in pumps, water heaters, kettles, in toilets or around taps. If you have hard water you may find you can see it deposited on glasses if they are left to dry after being washed.
Calcium/magnesium deposits are generally known as “scale”.
The removal of scale, known as “descaling”, has always been a major maintenance issue in steam engines, boilers for heating systems and power stations.
Problems caused by hard water
In household situations scale has a number of detrimental effects, most are merely inconvenience but some can cause expense.
- Hard water stops the soap from lathering properly making a shower a rather disappointing experience.
- Scale in your kettle coats the heating element and makes it inefficient. If the scale builds up too much your kettle will eventually fail requiring a new element or even a new kettle.
- Scale in your toilet can look very unsightly and may block up the valves in the cistern.
- In a water pump it can choke up the impellor.
- Scale can block water pipes.
- Scale can also leave a mat surface on the tiles in your bathroom. If under the shower the tiles are not shiny it is usually a thin film of calcium deposit although it could be fat from soap or a roughened surface caused by cleaning with a scouring agent.
- Probably the worst effect of scale deposit is in water heaters where it is deposited on the inner surface of internal water pipes. The internal diameter of the pipes is reduced which considerably reduces the heating efficiency of the heater but worse reduces the flow of water through the heater. This reduced flow is a nuisance and can reduce your shower to a mere dribble but, in instant demand type of gas and electric heaters scale can also stop the heater’s control system working properly. Eventually you have to replace the heater.
Hydrochloric Acid Removes Scale
So how do you remove calcium deposits? Calcium/magnesium deposits are very hard and trying to chip it off can be a bit of a disaster especially from fragile items such as heating elements. The usual method is to use hydrochloric acid which, quite worryingly, is freely available in many hardware shops. BE CAREFUL IT IS VERY NASTY STUFF. If you must use it use rubber gloves, protect your eyes, do not breathe in the fumes and be very careful where you dispose of it.
It is far better to avoid using it.
How do we make hard water soft?
It is not easy to remove the calcium/magnesium because it is in solution, it is fully disolved in the water.
Filtering will not remove the calcium or magnesium ions.
Distilling the water will remove the calcium/magnesium but this is an expensive exercise.
Water Softeners

The cheapest and easiest solution is to get a water softener.
A water softener has four parts:
- A cylinder full of plastic (resin) beads through which the water passes.
- A second cylinder full of salt water
- An automatic tap on the top of the first cylinder which from time to time flushes saltwater through the beads to remove deposits.
- A tank of salt water.
A water softener is basically a cylinder full of plastic beads which is installed in the water supply to your house. The plastic or “resin” beads are precharged with sodium ions. As the water flows through the cylinder it passes over the beads, the calcium and magnesium ions are exchanged for sodium ions which leave the calcium and magnesium deposited on the surface of the beads.
Every now and then (depending on how hard your water is) you regenerate the beads by “backwashing” using a strong salt water solution. The salt water solution (Sodium Chloride) flows from a second cylinder through the first one and ass it flows over the beads it displaces the calcium and magnesium that has built up on the beads replacing it with sodium ready to start again. The salt water flushes away the released calcium and magnesium deposits to a drain.
This is an environmentally safe process with only natural salt, calcium and magnesium being deposited in the drains.
Your standard water pressure is usually sufficient to push the water through a water softening unit.
There are many water softeners available from small “under the sink” mounted units designed just to soften the water for your kitchen sink to large household units that soften all your household supply to toilets, showers, water heaters and taps.
The process is quite simple and, while many fancy looking units are available for upwards of $1500, rugged practical models with automatic backwash can be found for around $600. It may sound a lot but for people who are struggling with a serious problem it is little price to pay. If you decide to move house you can take the water softener with you.
Note that water softeners use salt water to operate and this will leave your softened water with a slightly salty taste.
See also:
- Water causes stomach problems
- Water contamination and how it may be caused
- Water laboratory testing and how to read the results
- How to filter your water and contamination that can be removed
Phil Wilson
Copyright © Phil Wilson November 2007
This article, or any part of it, cannot be copied or reproduced without permission from the copyright owner.
